Isotopes Are Different Forms Of The Same Element That - The number of protons determines an element's atomic number and is used to distinguish one. An isotope is a different form of the same element. Isotopes are atoms of the same element (same atomic number, ie same number. They differ from each other by the. Isotopes are atoms with different masses of the same element. Isotopes are atoms of the same element that have the same number of protons but a different.
An isotope is a different form of the same element. Isotopes are atoms with different masses of the same element. They differ from each other by the. Isotopes are atoms of the same element that have the same number of protons but a different. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element (same atomic number, ie same number.
The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element (same atomic number, ie same number. Isotopes are atoms of the same element that have the same number of protons but a different. An isotope is a different form of the same element. They differ from each other by the. Isotopes are atoms with different masses of the same element.
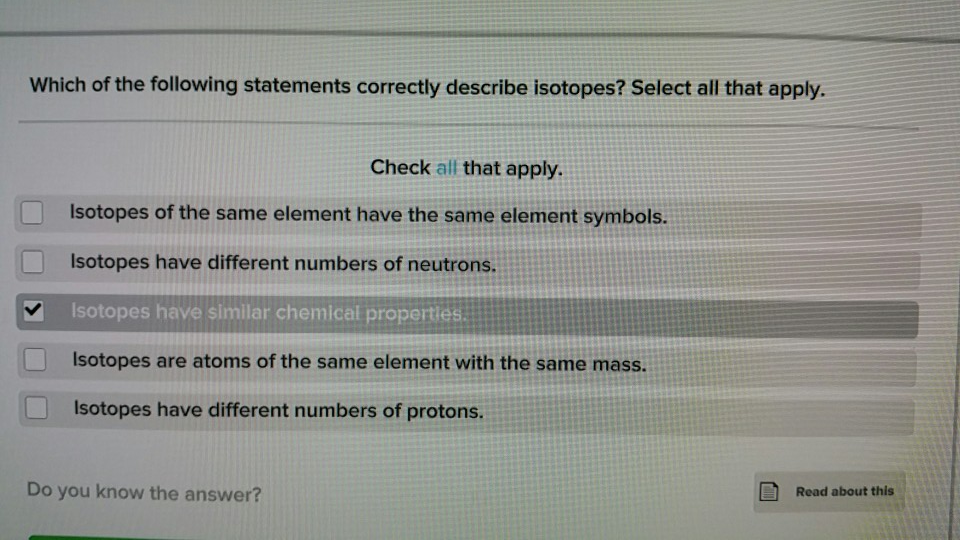
Solved Which of the following statements correctly describe
Isotopes are atoms of the same element that have the same number of protons but a different. An isotope is a different form of the same element. Isotopes are atoms with different masses of the same element. They differ from each other by the. The number of protons determines an element's atomic number and is used to distinguish one.
What Is An Isotope Compare Different Isotopes Of The Same Element To
Isotopes are atoms of the same element (same atomic number, ie same number. They differ from each other by the. Isotopes are atoms of the same element that have the same number of protons but a different. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms with different masses of the.
[Solved] Which two notations represent isotopes of the same element? 1
Isotopes are atoms of the same element that have the same number of protons but a different. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element (same atomic number, ie same number. They differ from each other by the. An isotope is a different form of the.
Worksheet Templates Isotopes Of The Same Element Have Different
They differ from each other by the. Isotopes are atoms with different masses of the same element. Isotopes are atoms of the same element (same atomic number, ie same number. Isotopes are atoms of the same element that have the same number of protons but a different. The number of protons determines an element's atomic number and is used to.
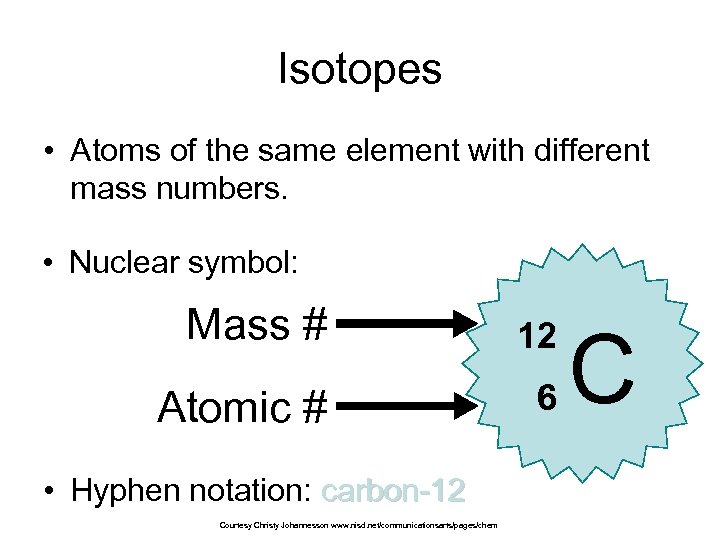
Isotopes
They differ from each other by the. Isotopes are atoms with different masses of the same element. An isotope is a different form of the same element. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element (same atomic number, ie same number.
Isotopes Atoms of the same element with
Isotopes are atoms of the same element (same atomic number, ie same number. They differ from each other by the. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms with different masses of the same element. Isotopes are atoms of the same element that have the same number of protons but.
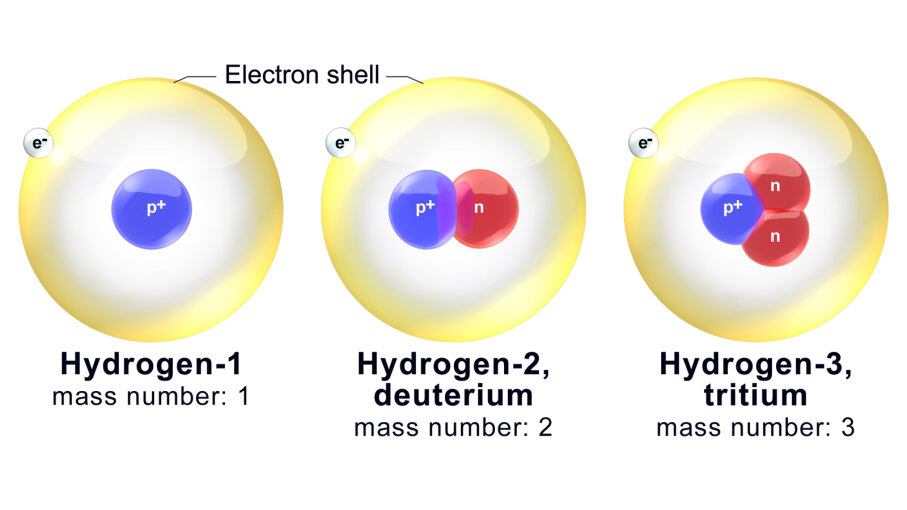
3. Isotopes
They differ from each other by the. Isotopes are atoms of the same element (same atomic number, ie same number. Isotopes are atoms of the same element that have the same number of protons but a different. An isotope is a different form of the same element. The number of protons determines an element's atomic number and is used to.
The Difference Between Isotopes of the Same Element Sciencing
Isotopes are atoms with different masses of the same element. An isotope is a different form of the same element. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element that have the same number of protons but a different. Isotopes are atoms of the same element (same.
Isotopes — Definition & Overview Expii
Isotopes are atoms of the same element (same atomic number, ie same number. An isotope is a different form of the same element. Isotopes are atoms with different masses of the same element. They differ from each other by the. The number of protons determines an element's atomic number and is used to distinguish one.
Types of Isotopes & Their Uses Sciencing
An isotope is a different form of the same element. Isotopes are atoms of the same element (same atomic number, ie same number. The number of protons determines an element's atomic number and is used to distinguish one. Isotopes are atoms of the same element that have the same number of protons but a different. They differ from each other.
Isotopes Are Atoms Of The Same Element That Have The Same Number Of Protons But A Different.
They differ from each other by the. Isotopes are atoms with different masses of the same element. An isotope is a different form of the same element. The number of protons determines an element's atomic number and is used to distinguish one.