Differential Rate Law - The differential rate law can be. Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt). In other words, if we have a reaction of the type: In general, a rate law (or differential rate law, as it is sometimes called) takes this form: A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). They are used to describe what is.
Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt). The differential rate law can be. A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). In other words, if we have a reaction of the type: They are used to describe what is. Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of.
In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if we have a reaction of the type: Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). They are used to describe what is. The differential rate law can be. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt).
Rate Law and Integrated Rate Law Diagram Quizlet
In other words, if we have a reaction of the type: Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; They are used to describe what is. A differential rate law expresses the reaction rate in terms of changes in the.
Solved From the differential rate law for a secondorder
Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. In other words, if we have a reaction of the type: Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; Differential rate laws are used to express.
Solved 2. Differential rate law relates instantaneous
In other words, if we have a reaction of the type: In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. They are used to describe what is. A differential rate law expresses the reaction rate in terms of.
Integrated Rate Law Chemistry Steps
Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. In other words, if we have a reaction of the type: A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). They are used to describe what is. In.
Integrated Rate Law Chemistry Steps
Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt). They are used to describe what is. The differential rate law can be. In other words,.
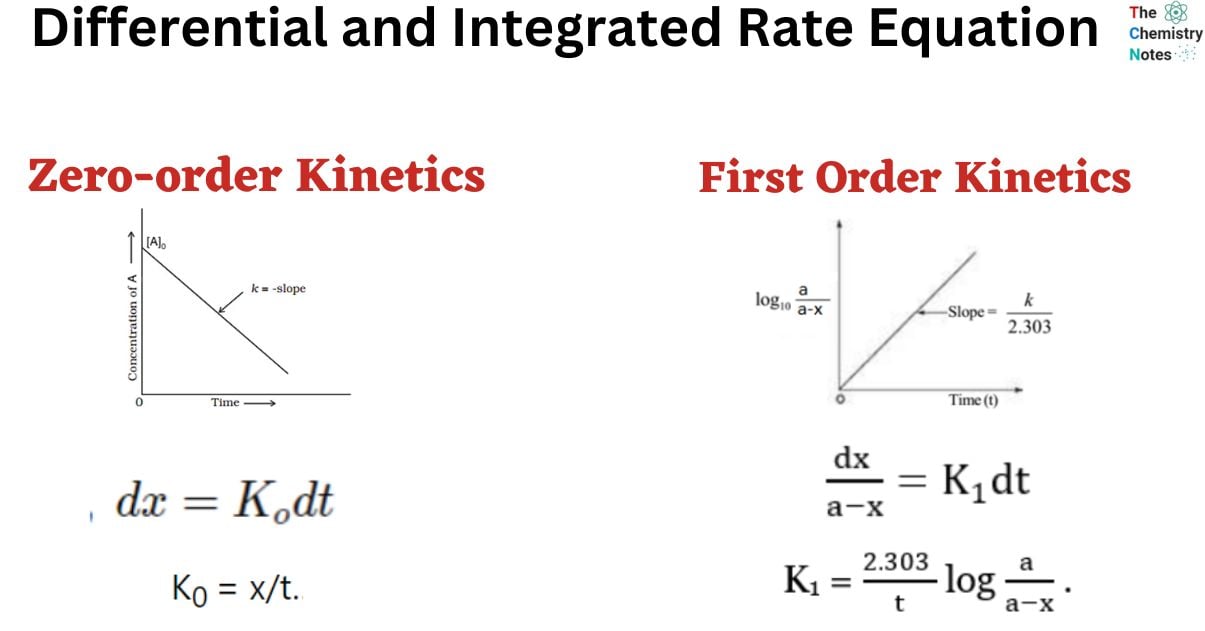
Differential and Integrated Rate Equation
In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if we have a reaction of the type: They are used to describe what is. Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. Differential rate laws are used to express the rate of a.
The type of rate law for a reaction, either the d…
A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). In other words, if we have a reaction of the type: The differential rate law can be. Differential rate laws express the rate of reaction as a function of a change in the.
Differential Rate Law Equation Method… Chemistry in Hindi
Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. The differential rate law can be. Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time; Differential rate laws are used to express the rate of a reaction.
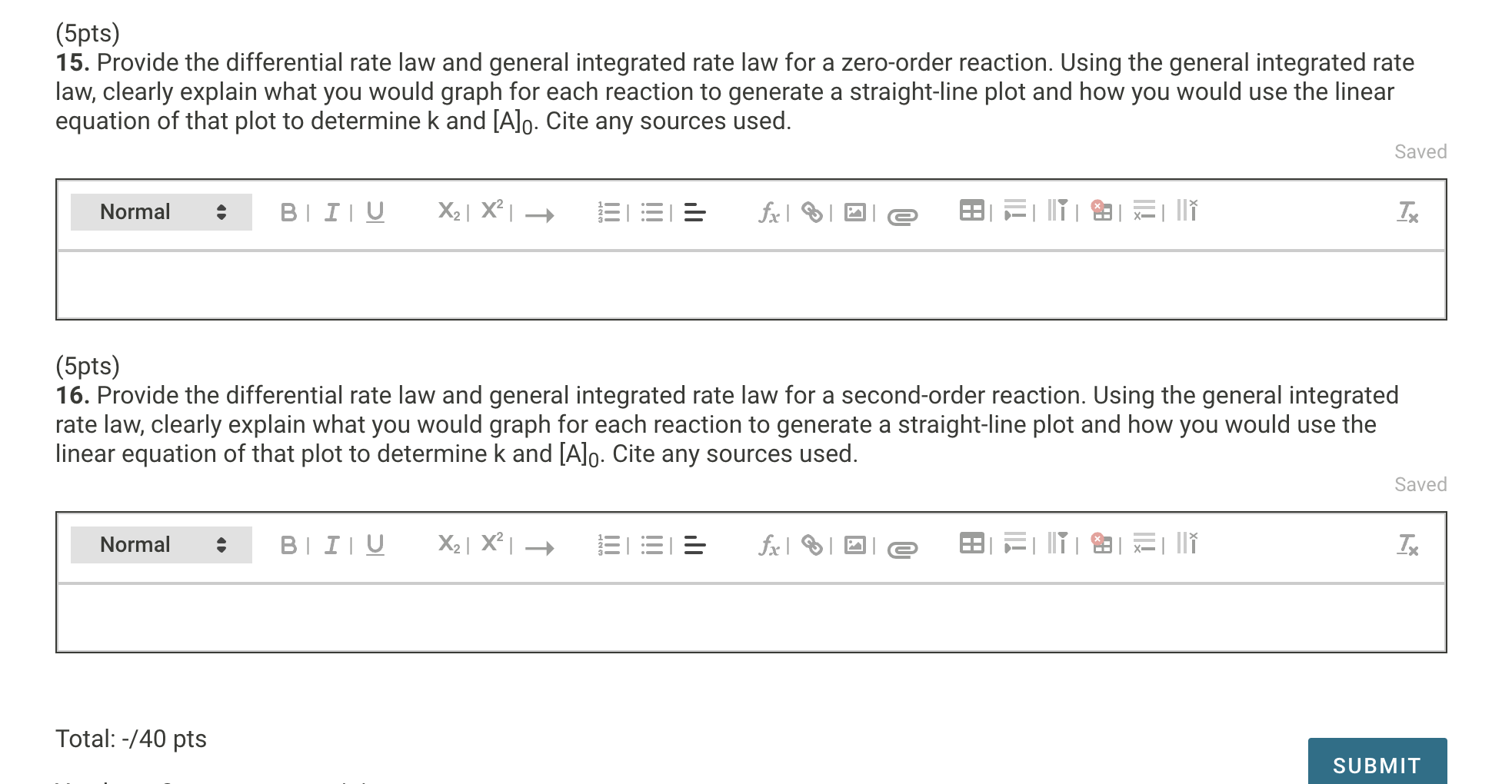
Solved (5pts) 15. Provide the differential rate law and
Rate = k[a]m[b]n[c]p… in which [a], [b], and [c] represent the molar concentrations of. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt). In general, a rate law (or differential rate law, as it is sometimes called) takes this.
Differential Rate Equation
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration of reactants (d [r]) over a small interval of time (dt). In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Differential rate laws express the rate of reaction as a function of a.
Rate = K[A]M[B]N[C]P… In Which [A], [B], And [C] Represent The Molar Concentrations Of.
They are used to describe what is. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: A differential rate law expresses the reaction rate in terms of changes in the concentration of one or more reactants (δ[r]) over a specific time interval (δt). The differential rate law can be.
Differential Rate Laws Are Used To Express The Rate Of A Reaction In Terms Of Change In The Concentration Of Reactants (D [R]) Over A Small Interval Of Time (Dt).
In other words, if we have a reaction of the type: Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time;