Differential Rate Law For Zero Order Reaction - Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Given the following two reactions and their experimentally determined differential rate laws:. There are three commonly encountered differential rate laws.
Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Given the following two reactions and their experimentally determined differential rate laws:. There are three commonly encountered differential rate laws. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law.
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Given the following two reactions and their experimentally determined differential rate laws:. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law.
Zeroorder Reaction Rate Equation, Unit, Graph, Example
Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Given the following two reactions and their experimentally determined differential rate laws:. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Zeroth order reactions ( j=0) the differential form of.
For a zeroorder reaction,
Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. There are three commonly encountered differential rate laws. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Given the following.
100 1 21 What is zero order reaction? Write differential rate law the
Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Given the following two reactions and their experimentally determined differential rate laws:. There are three commonly encountered differential rate laws. Differential rate laws are used to express the rate of.
Rate Constant Equation For Zero Order Reaction Tessshebaylo
Given the following two reactions and their experimentally determined differential rate laws:. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Either the differential.
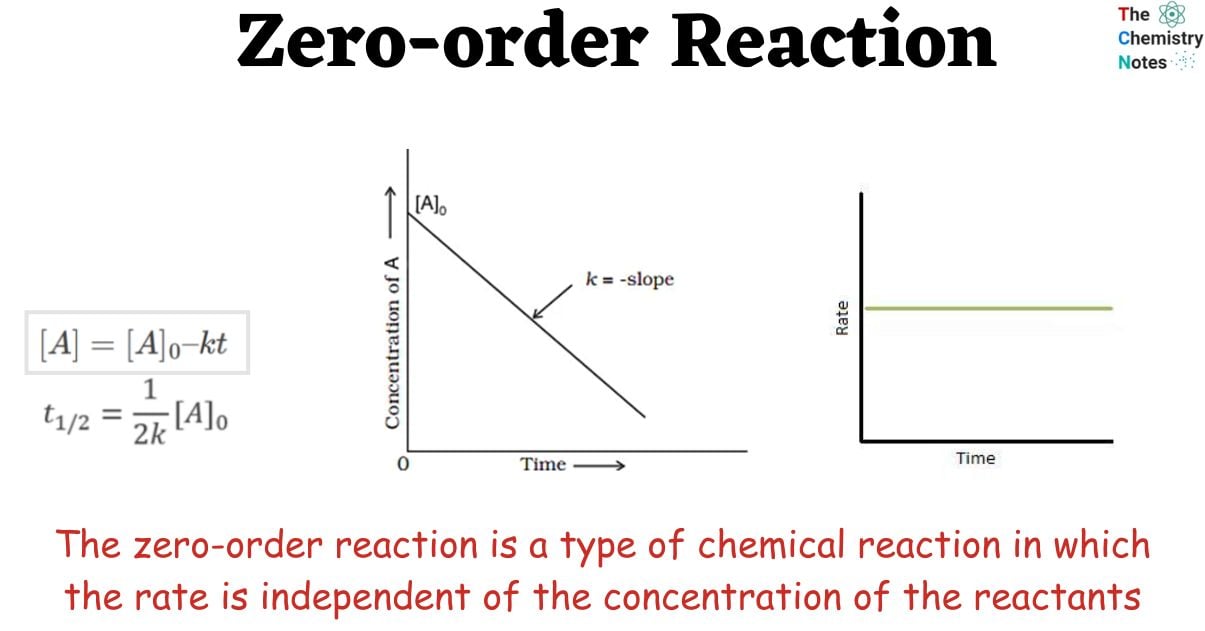
Zero order reaction Definition, integrated rate law, characteristics
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Given the following two reactions and their experimentally determined differential rate laws:. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. There are three commonly encountered differential rate laws. Either the differential.
Rate Constant Equation For Zero Order Reaction Tessshebaylo
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Given the following two reactions and their experimentally determined differential rate laws:. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of.
Zero Order Reaction Equation Hot Sex Picture
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Given the following two reactions and their experimentally determined differential rate laws:. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Either the differential.
100 1 21 What is zero order reaction? Write differential rate law the
There are three commonly encountered differential rate laws. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Given the following two reactions and their experimentally determined differential rate laws:. Either the differential.
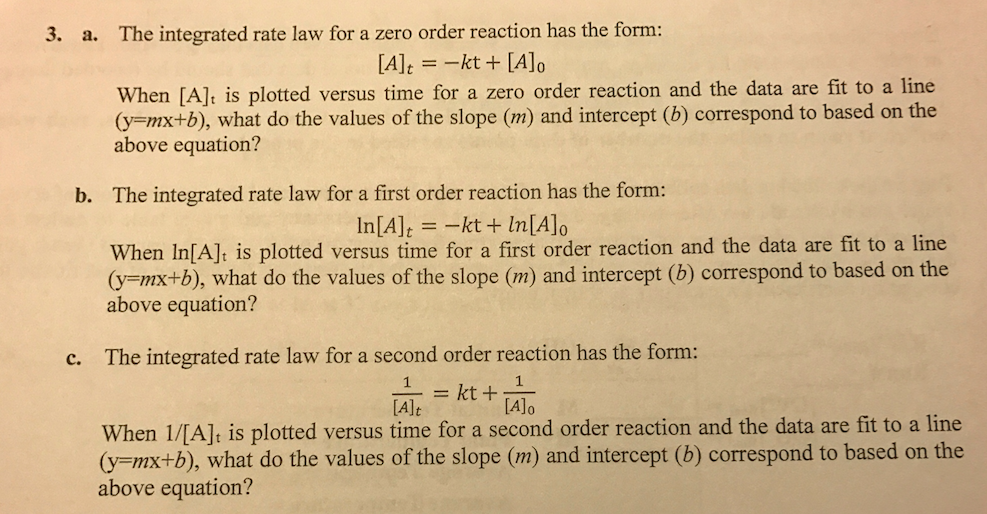
Solved The integrated rate law for a zero order reaction has
Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Given the following two reactions and their experimentally determined differential rate laws:. Either the differential.
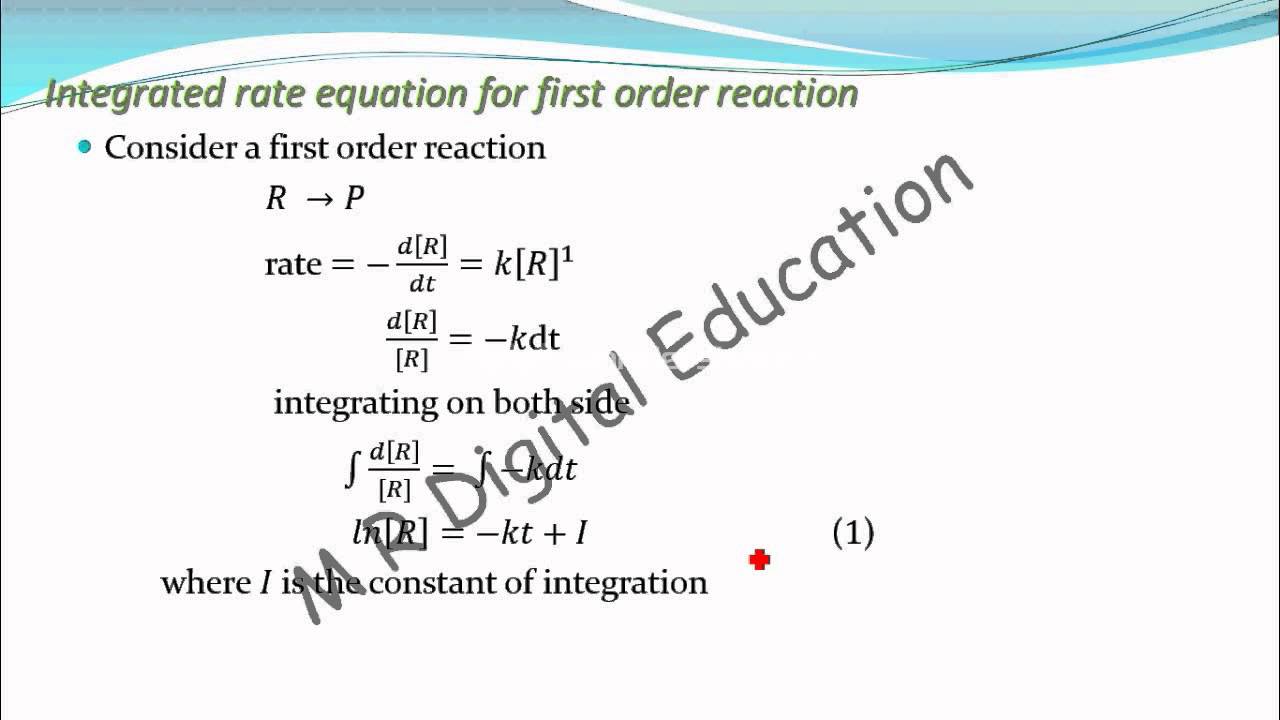
24++ Derive The Integrated Rate Law For First Order Reaction Marcodd
Given the following two reactions and their experimentally determined differential rate laws:. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of.
Given The Following Two Reactions And Their Experimentally Determined Differential Rate Laws:.
Zeroth order reactions ( j=0) the differential form of the rate law is (notice the presence of the. Differential rate laws are used to express the rate of a reaction in terms of change in the concentration. There are three commonly encountered differential rate laws. Either the differential rate law (equation \(\ref{14.4.5}\)) or the integrated rate law.