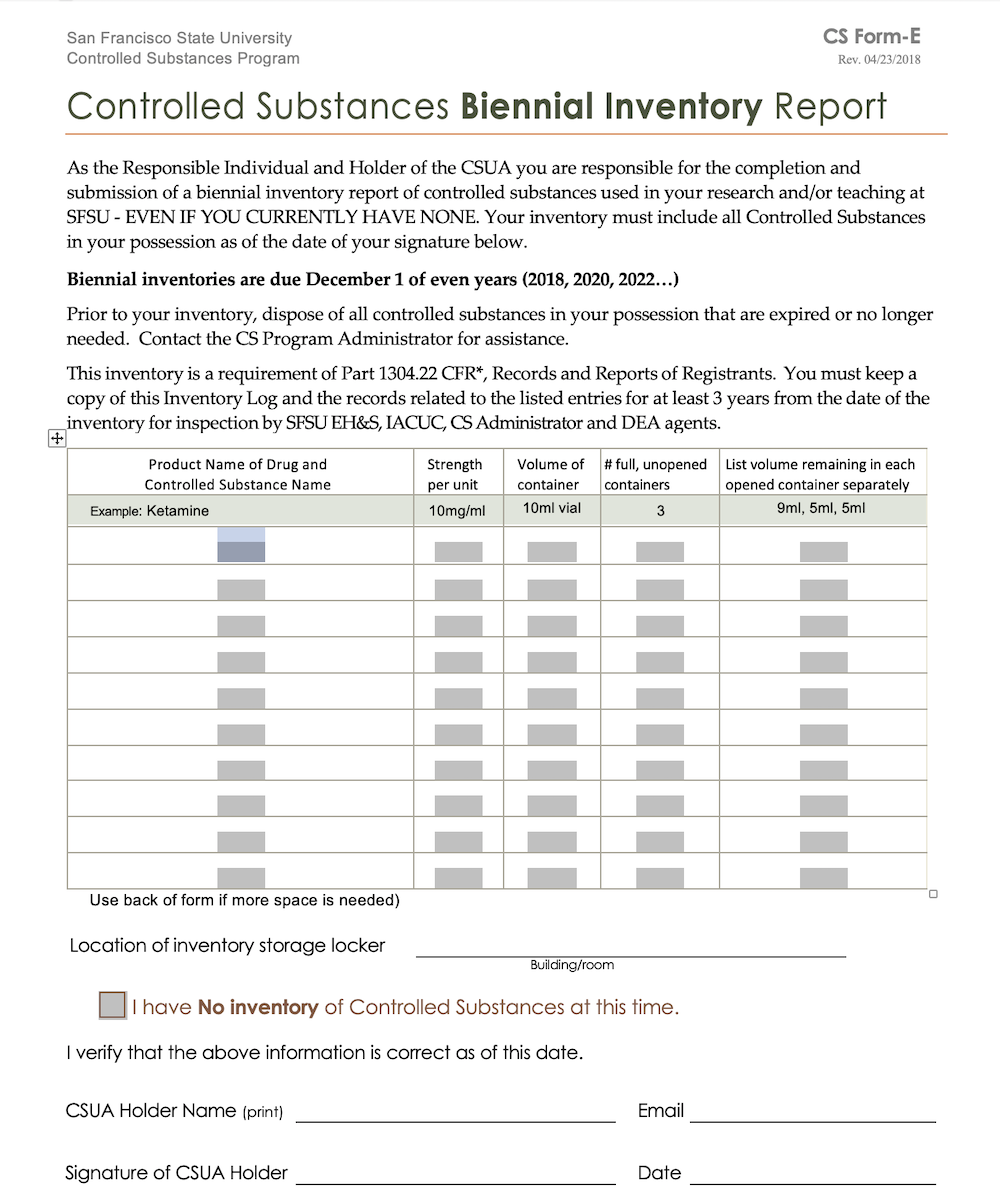
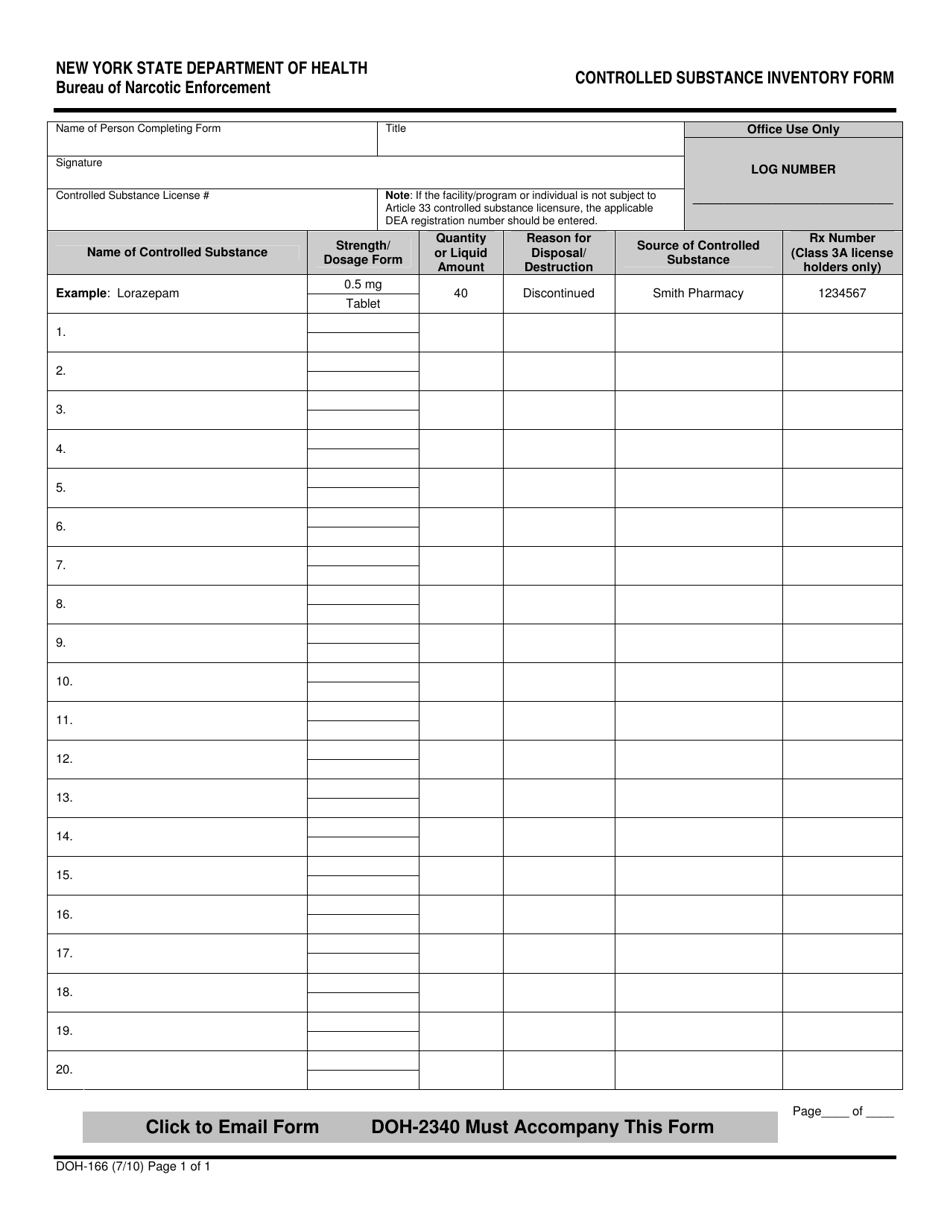
Dea Biennial Inventory Form - (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);
Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each.
The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or.
DEA Biennial Controlled Substance Inventory Form Fill and Sign
(2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must.
CS Form E Biennial Inventory Environment, Health and Safety
(2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other.
Dea form 41 Fill out & sign online DocHub
Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in.
Dea biennial inventory form Fill out & sign online DocHub
Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted.
SOLUTION Cs biennial inventory form Studypool
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. Modify eligible dea registration to.
5 Best Printable Home Med Printablee 15048 Hot Sex Picture
The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must.
Form DOH166 Fill Out, Sign Online and Download Fillable PDF, New
The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered.
Dea Controlled Substance Log Template JMT Printable Calendar
(2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii.
Fillable Online DEA Controlled Substance Inventory Form Fax Email Print
(2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must.
Fillable Online Biennial & Initial Controlled Substance Inventory Form
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances.
Modify Eligible Dea Registration To Collect Pharmaceutical Controlled Substances From Ultimate Users (E.g., Patients);
(2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location.